AstraZeneca is retracting its Covid-19 vaccine after being in the market in less than four years following its launch in the UK. This decision comes as a result of decreased demand for the vaccine due to an excess of medications focusing on newer variants.
The pharmaceutical company announced on Wednesday that it takes pride in the contribution of Vaxzevria in combatting the global pandemic that has been affecting globally. However, the company has ceased production and supply of the product due to an excess availability of updated vaccines.

This decision marks the conclusion of the journey for the vaccine, which was developed in partnership with scientists from Oxford University within months of the pandemic’s onset. Approved in the UK in late 2020, over 3 billion doses have been supplied since its inception.
In contrast to competitors like Pfizer, BioNTech, and Moderna, AstraZeneca initially embraced a non-profit approach for its vaccine, selling it “at cost” in alignment with its agreement with Oxford University. Despite the pivotal role the drug played in halting the pandemic, its rollout faced challenges, including rare instances of blood clotting and disputes with the European Union regarding access to the vaccine.
AstraZeneca reported that independent estimates indicate over 6.5 million lives were saved in the first year of its vaccine’s usage alone. However, the company acknowledged that the development of multiple Covid-19 vaccines since then has affected sales of its own product.
The announcement follows the pharmaceutical group’s request in March for the European Union marketing authorisation for Vaxzevria to be withdrawn, a request that was granted on Tuesday.
AstraZeneca’s vaccine has been overtaken by shots from BioNTech/Pfizer and Moderna, which utilise mRNA technology and have emerged as the most widely used vaccines globally.
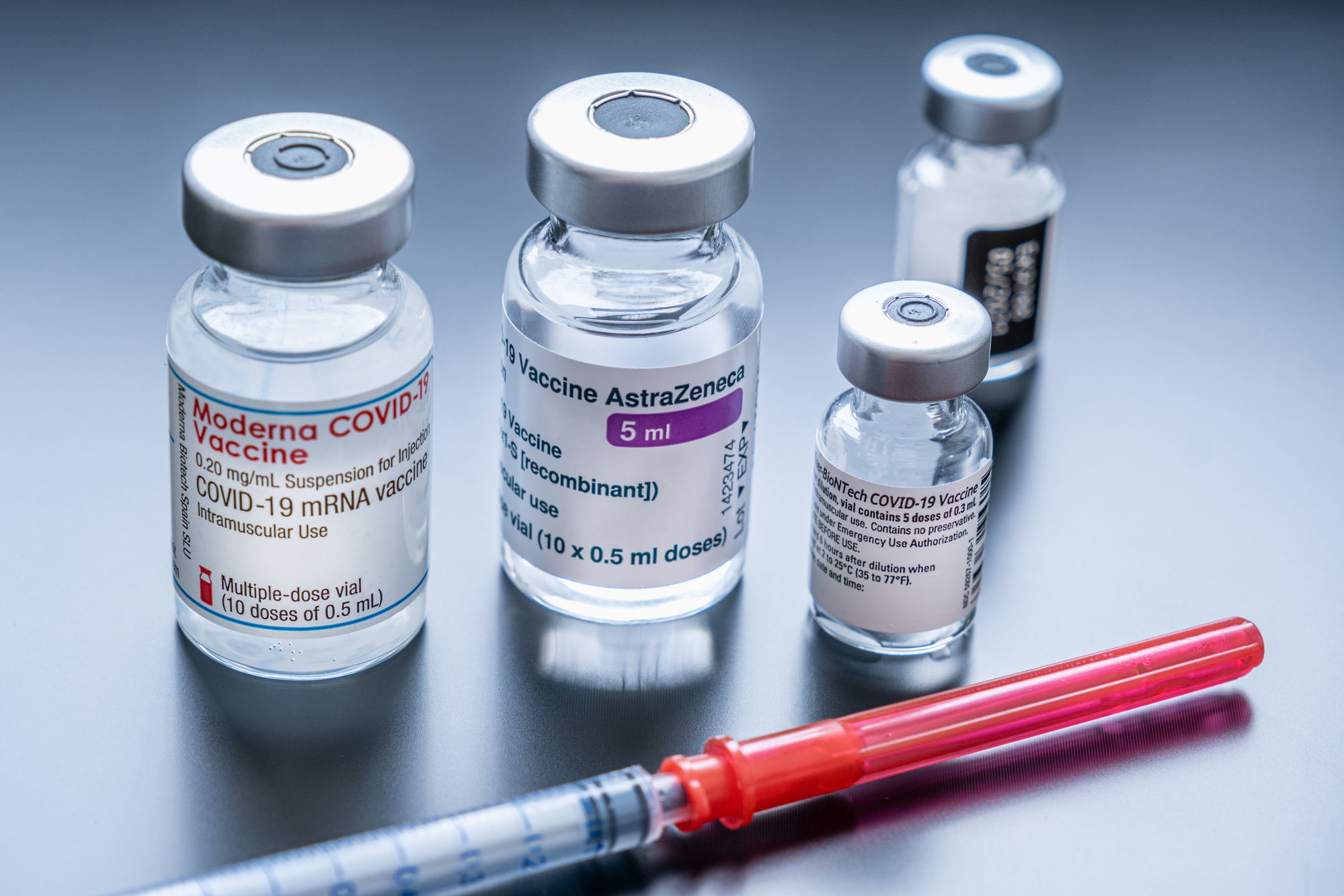
According to the company’s full-year results, AstraZeneca’s jab generated only $12 million in revenue in 2023, a significant drop from nearly $4 billion in 2021. AstraZeneca began signing its first for-profit deals in late 2021, stating that the pandemic had transitioned into an “endemic phase.”
The vaccine gained approval in the UK in December 2020 and in the EU in January 2021. However, it never received approval in the US, where authorities criticised the way, the company presented data on the efficacy of its vaccine.
While the vaccine was deemed safe and effective overall, confidence in it wavered in 2021 following a series of rare blood-clotting incidents. As a result, European authorities restricted its usage among younger populations.
The company is facing a legal case from Jamie Scott, who alleges that he developed a severe blood clot as a result of taking the vaccine. If found liable, any damages would be covered by a UK government vaccine damage payment scheme. However, the company stated that the withdrawal of the vaccine was not connected to the rare blood-clotting incidents.

AstraZeneca stated, “We will now collaborate with regulators and our partners to establish a clear path forward to bring closure to this chapter and acknowledge our significant contribution to the Covid-19 pandemic.”


