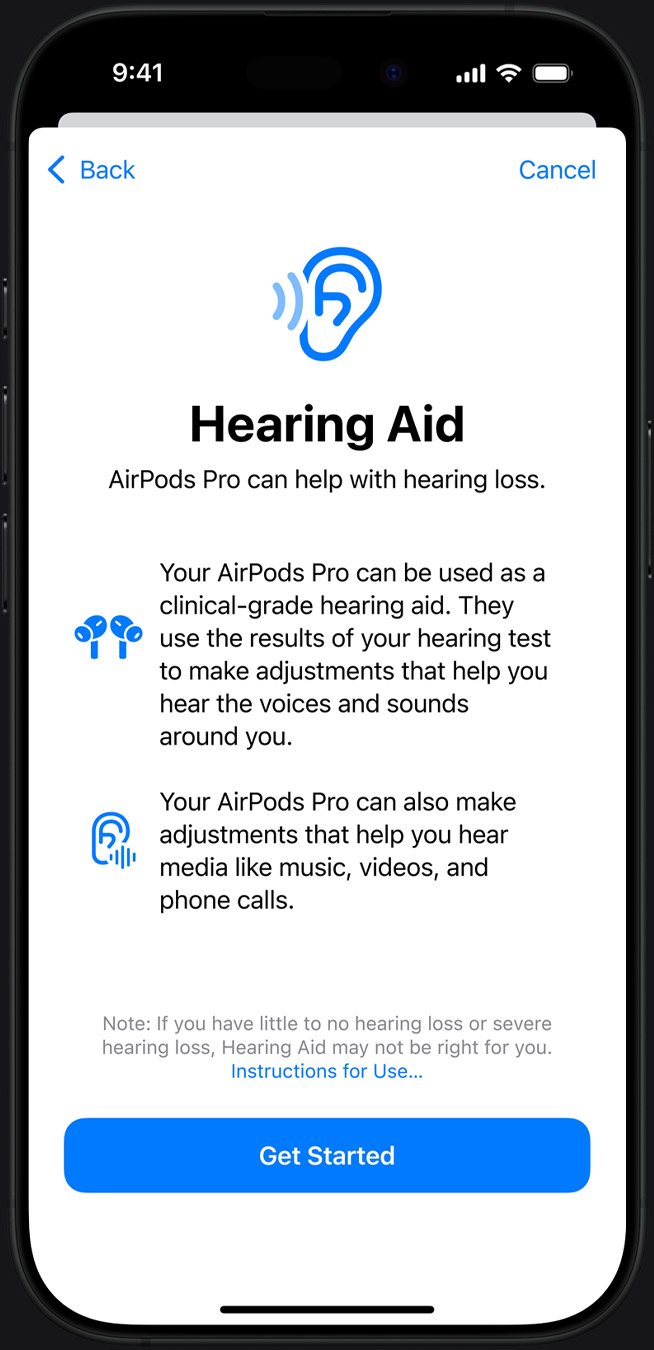
Apple’s popular AirPods Pro has recently gained FDA approval as an over-the-counter hearing aid software. This innovative feature will soon be available on compatible devices.

Just days after Apple’s “Glowtime” event, where they introduced various new health features, the FDA officially approved the company’s Hearing Aid Feature (HAF). This groundbreaking feature enables AirPods Pro 2 users to conduct a personalised hearing test directly on their device.
Additionally, the test results are then used to tailor the audio settings in the hearing assistance software, effectively transforming the AirPods Pro into a customised form of hearing aid.
A little throwback during its September 10 event, Apple introduced the Hearing Aid mode for AirPods Pro 2, anticipating that the FDA would approve it shortly. However, to Apple’s surprise, the FDA granted approval much faster than expected.
Furthermore, Michelle Tarver, Director of the FDA’s Center for Devices and Radiological Health, announced today a significant milestone in hearing assistance. The FDA, with its rigorous evaluation process, has granted marketing authorisation for over-the-counter hearing aid software on a widely used consumer audio device, a pivotal step in improving the availability, accessibility, and acceptability of hearing assistance for adults with perceived mild to moderate hearing loss.
The FDA’s approval also shed light on the clinical testing of Apple’s HAF. According to the agency, the feature was tested in a study involving “118 subjects with perceived mild to moderate hearing loss across multiple U.S. sites.” The results showed that participants using the HAF’s self-fitting strategy experienced a similar perceived benefit to those with professionally fitted devices.
Not only that, tests measuring amplification levels in the ear canal and speech understanding in noisy environments yielded comparable results. The FDA noted that no adverse events related to the device were observed during the study.
The consumer enthusiasm for Apple’s Hearing Aid mode strengthens the FDA’s recent 2022 regulations regarding over-the-counter hearing aids. These regulations, which allow individuals with mild to moderate hearing impairment to buy hearing aids without undergoing a medical examination, prescription, or audiologist approval, have received positive reception from consumers.
AirPods Pro users can look forward to the Hearing Aid mode, which will be introduced in a forthcoming software update for compatible devices.
With the FDA’s approval, Apple’s Hearing Aid Feature is poised to revolutionise the way people with hearing loss access and utilise hearing aids. Its blend of technology and accessibility is a testament to Apple’s commitment to improving people’s lives.


